
Whether working in batch or flow, most chemists encounter challenges when performing difficult liquid-liquid/gas extractions. Zaiput Flow Technologies’ patented liquid-liquid/gas-liquid separators solve the most difficult separation problems with ease, backed by solid, proven results.
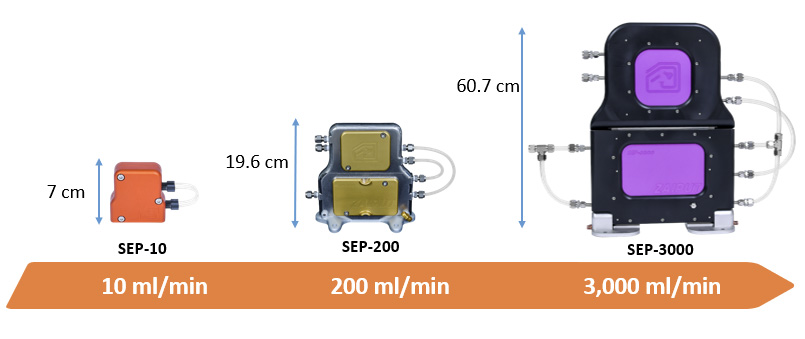
For larger scales separators click on this link: PRODUCTION SCALE SEPARATORS AND EXTRACTION PLATFORMS
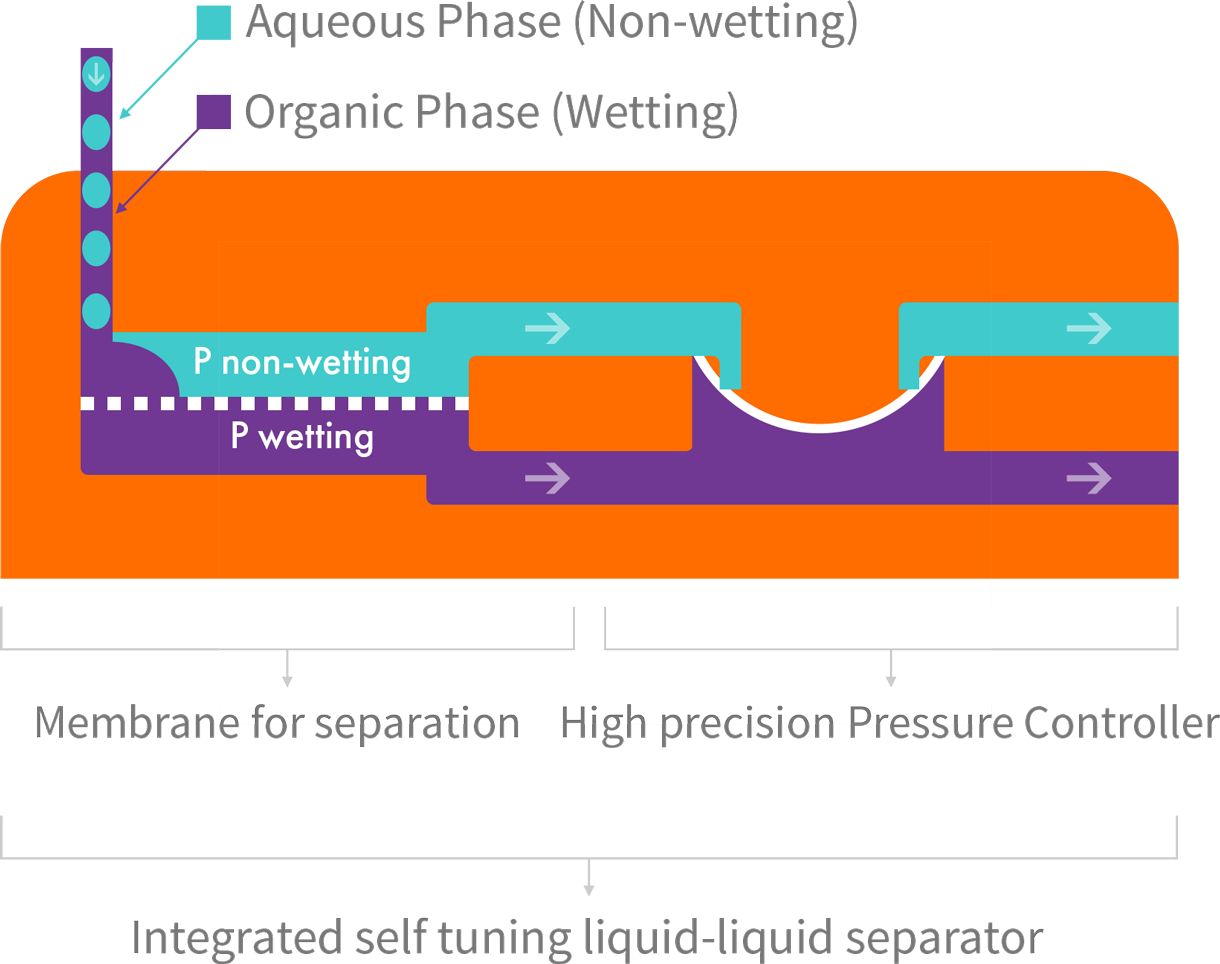
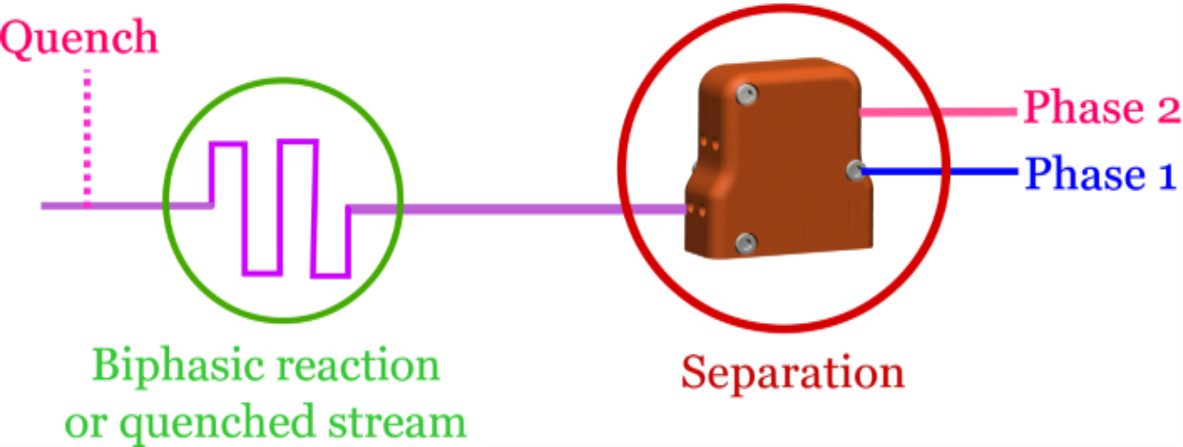
Zaiput Flow Technologies’ patented separators provide continuous separation of an immiscible phase (liquid-liquid or gas-liquid) by leveraging differences in wetting properties of the liquids onto a porous membrane:
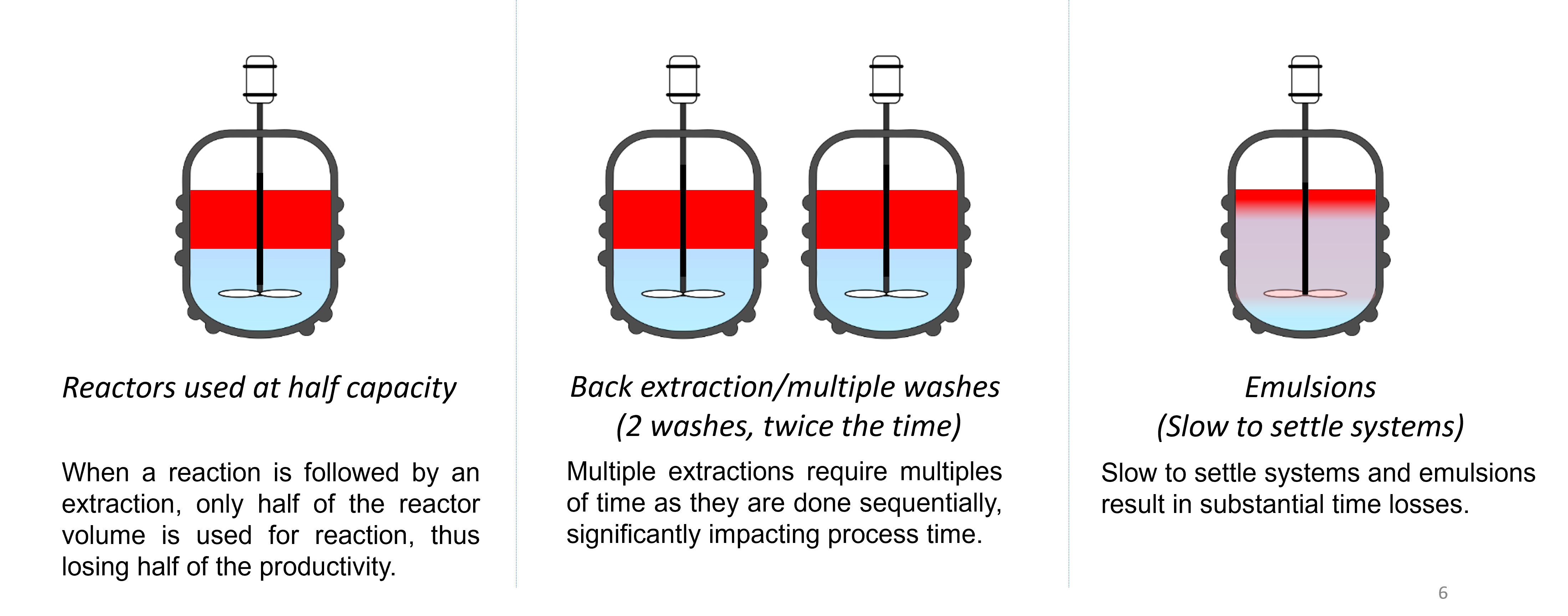
Our separators provide simple solutions to significant problems including: separation of emulsions, elimination of the need to run batches at half capacity and slow settling time, to name a few. Let’s look at the details of issues that may be encountered without our technology when working in batch or flow:
The modern approach to chemical manufacturing seeks to continuously leverage the advantages of continuous manufacturing
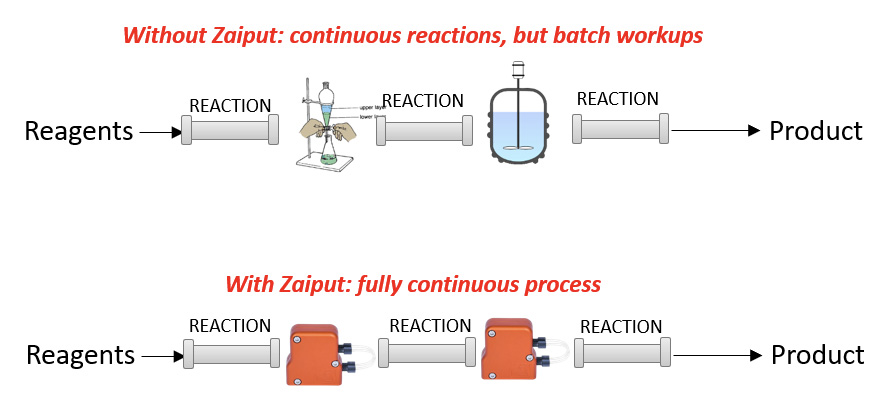
Before Zaiput separators existed, only a piecewise continuous process existed due to the lack of inline separation capabilities.
If the video does not play properly, click here to see our demo.
Zaiput Flow Technologies’ patented separators provide continuous separation of an immiscible phase (liquid-liquid or gas-liquid) by leveraging differences in wetting properties of the liquids onto a porous membrane.

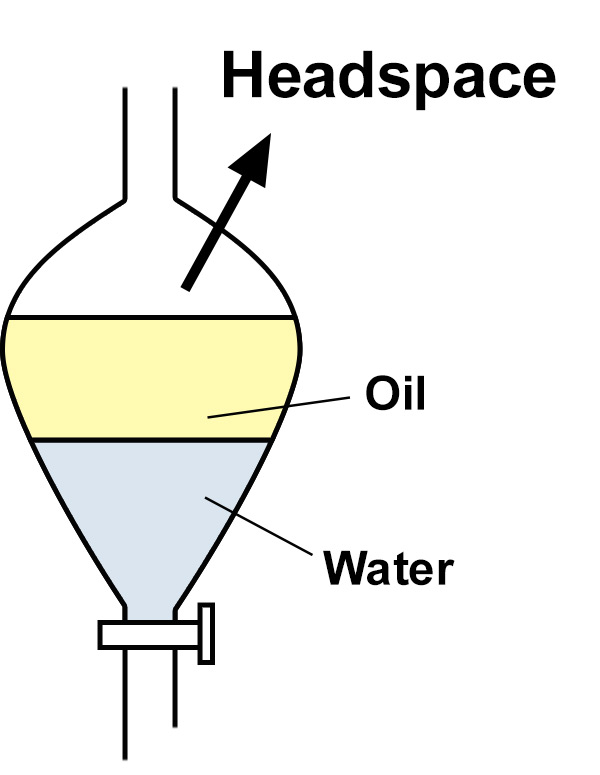
separation of liquids on a membrane
Separator Function: When a hydrophobic membrane is in place, the organic, which is the wetting phase (purple), passes through the membrane (dotted line) while the aqueous, which is the non-wetting phase (blue), is retained.
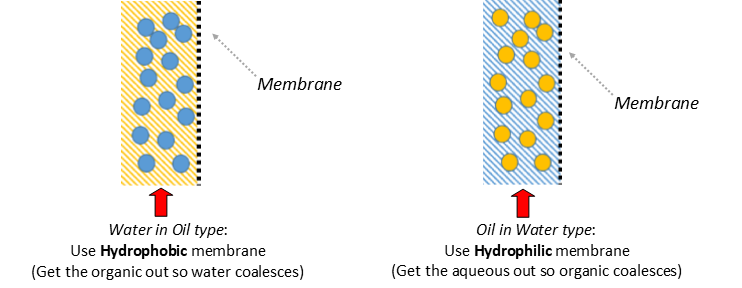
membrane separation can be done with either a hydrophilic or hydrophobic membrane
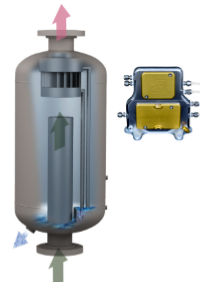
membrane-based liquid liquid separators require a much smaller footprint

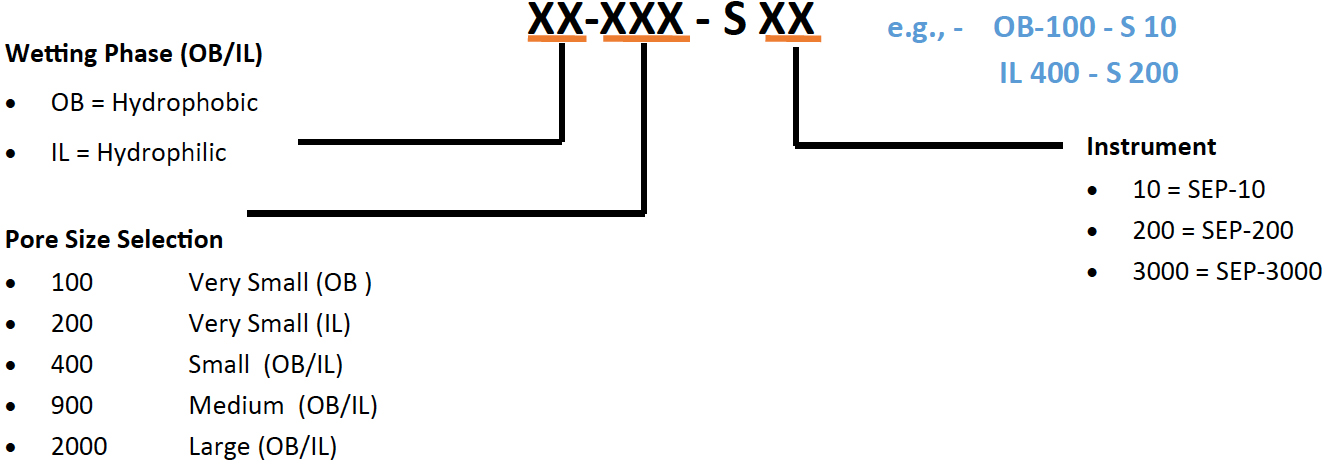
A variety of membranes for your Zaiput separator are available to optimize separation performance and throughput. Membranes are available in both hydrophobic and hydrophilic, they are low cost and easy to replace.
Membrane selection process:
The key parameters for identifying a suitable membrane for separation are the interfacial tension between the two phases and the viscosity of the permeating phase (this has an effect on throughput of the device).
In general, the lower the interfacial tension, the smaller the pore size needs to be. However, smaller pore size reduces the maximum viscosity that can be accommodated by the membrane.
>> Membrane Sampler Package : MEM-10 (available on request for larger devices) – 7 packs of 10 membranes
>> Quick Start Package option : START-10 ( membrane samples MEM-10 + replacement diaphragm)
>> Specific membrane ordering info:
Contact us for the latest membrane offering
For general applications, we recommend reviewing the information in the Membrane Selection Guide.
If you have any questions, contact us at support@zaiput.com
Yiota-Victoria Phakoukaki, Paul O’Shaughnessy, Panagiota Angeli, Intensified liquid-liquid
extraction of biomolecules using ionic liquids in small channels, Separation and
Purification and Technology, Feb 2022.
David Roura Padrosa and Martina L. Contente, Multi-gram preparation of
cinnamoyl tryptamines as skin whitening agents through a chemo-enzymatic flow process,
Tetrahedron Letters, Dec 2021.
Cecilia Pinna, Piera Anna Martino, Gabriele Meroni, Valerio Massimo Sora, Lucia Tamborini, Sabrina
Dallavalle, Martina L. Contente, and Andrea Pinto, Biocatalyzed Synthesis of Vanillamides and
Evaluation of Their Antimicrobial Activity, J. Agric. Food Chem., Dec
2021.
Francesca Annunziata, Alessandra Guaglio, Paola Conti, Lucia Tamborini and Raffaella Gandolfi, Continuous-flow
stereoselective reduction of prochiral ketones in a whole cell bioreactor with natural deep eutectic
solvents, Green Chemistry, Dec 2021.
Mara Di Filippo and Marcus Baumann, Continuous Flow
Synthesis of Anticancer Drugs, Molecules, Nov 2021.
Francesca Annunziata, Martina L. Contente, Cecilia Pinna, Lucia Tamborini, and Andrea Pinto, Biocatalyzed Flow Oxidation of Tyrosol to Hydroxytyrosol and
Efficient Production of Their Acetate Esters, Antioxidants, July 2021.
Jinu Joseph John, Tom Van Gerven, Effect of ultrasound on
parallel flow in a microchannel, Chemical Engineering and Processing – Process
Intensification, May 2021.
Itziar Peñafiel, Sebastian C. Cosgrove, Biocatalysis in Flow for Drug Discovery,
Flow Chemistry in Drug Discovery, April 2021.
Dimitrios Tsaoulidis, Milan Mamtora, Miguel Pineda, Eric S.Fraga, and Panagiota Angeli, Experimental and CFD
scale-up studies for intensified actinide/lanthanide separations, Chemical Engineering and
Processing – Process Intensification, Mar 2021.
Jorge García-Lacuna, Tobias Fleiß, Rachel Munday, Kevin Leslie, Anne O’Kearney-McMullan, Christopher A. Hone, and C.
Oliver Kappe, Synthesis of the Lipophilic
Amine Tail of Abediterol Enabled by Multiphase Flow Transformations, Org. Process
Res. Dev., Mar 2021.
Victor-Emmanuel H. Kassin, Romain Morodo, Thomas Toupy, Isaline Jacquemin, Kristof Van Hecke, Raphaël Robiette,
and Jean-Christophe M. Monbaliu, A modular, low footprint
and scalable flow platform for the expedient α-aminohydroxylation of enolizable ketones,
Green Chemistry, Feb 2021.
Micol Santi, Luca Sancineto, Vanessa Nascimento, Juliano Braun Azeredo, Erika V. M. Orozco, Leandro H. Andrade,
Harald Gröger, and Claudio Santi, Flow Biocatalysis: A
Challenging Alternative for the Synthesis of APIs and Natural Compounds, International Journal of
Molecular Sciences, Jan 2021.
Mellisa B. Sagandira, Cloudius R. Sagandira, and Paul Watts, Continuous flow synthesis of xylidines
via biphasic nitration of xylenes and nitro-reduction, Journal of Flow Chemistry, Jan
2021.
Glen Surjadinata, Luke Hunter, Lidia Matesic, and Giancarlo Pascali, Analytical-scale synthesis of
aryl-SF4Cl via flow microfluidic technology, Journal of Flow Chemistry., Jan 2021.
Samuel J. Miller, Haruro Ishitani, Yuichi Furiya, and Shu̅ Kobayashi, High-Throughput Synthesis of
(S)-α-Phellandrene through Three-Step Sequential Continuous-Flow Reactions, Org. Process Res.
Dev., Jan 2021.
Jillian W. Sheeran, Kiersten Campbell, Christopher P. Breen, Gerald Hummel, Changfeng Huang, Anamika Datta, Serge H.
Boyer, Scott J. Hecker, Matthew M. Bio, Yuan-Qing Fang, David D. Ford, and M. Grace Russell, Scalable On-Demand Production of Purified
Diazomethane Suitable for Sensitive Catalytic Reactions, Org. Process Res. Dev., Jan
2021.
Dimitrios Tsaoulidis, Milan Mamtora, Marta Mayals Gañet,
Eduardo Garciadiego-Ortega, and Panagiota Angeli,
Scale-Up Studies for Co/Ni Separations in Intensified Reactors, Micromachines., Dec
2020.
Hoang Khang Bui, Ki Yoon Kim, Hyeonjin Kim, Jae‐Pyoung Ahn, Taekyung Yu, and Tae Seok Seo, Total Integration of the Sample
Injection, Microdroplet Reaction, Phase Separation, Real‐Time Optical Detection, and Recovery of Diverse
Silver–Gold Bimetallic Nanoalloys in a Continuous Process,Particle., Dec 2020.
Su Wang, Yeersen Patehebieke, Zheng Zhou, Zhibing Zhang, and Xiao Wang, Recent
Advances in Continuous-Flow Reactions Using Metal-Free Homogeneous
Catalysts,Catalysts, Nov 2020.
Piera De Santis, Lars-Erik Meyer, and Selin Kara, The rise of continuous flow
biocatalysis – fundamentals, very recent developments and future perspectives, Reaction Engineering
and Chemistry, Oct 2020.
Naoto Sugisawa, Hiroyuki Nakamura, and Shinichiro Fuse, Catalyst-free biphasic oxidation of
Thiophenes in continuous-flow, Journal of Flow Chemistry, Jul 2020.
Jorge García‐Lacuna, Prof. Dr. Gema Domínguez, and Prof. Dr. Javier Pérez‐Castells, Flow Chemistry for
Cycloaddition Reactions, ChemSusChem, Jul 2020.
Elizabeth Farrant Automation of
Synthesis in Medicinal Chemistry: Progress and Challenges, ACS Med. Chem. Lett., Jul
2020.
Alejandro Mata, Ulrich Weigl, Oliver Flögel, Pius Baur, Christopher A. Hone and C. Oliver Kappe, Acyl azide generation and amide bond
formation in continuous-flow for the synthesis of peptides, React. Chem. & Eng.,
Mar 2020.
Kristina Søborg Pedersen, Christina Baun, Karin Michaelsen Nielsen, Helge Thisgaard, Andreas Ingemann Jensen and
Fedor Zhuravlev, Design,
Synthesis, Computational, and Preclinical Evaluation of natTi/45Ti-Labeled Urea-Based Glutamate PSMA
Ligand, Molecules, Mar 2020.
Peter Sagmeister, Johannes Poms, Jason Williams and C. Oliver Kappe, Multivariate analysis
of inline benchtop NMR data enables rapid optimization of a complex nitration in flow, React. Chem.
& Eng., Feb 2020.
Adam D. Clayton, Luke A. Power, William R. Reynolds, Caroline Ainsworth, David R. J. Hose, Martin F. Jones, Thomas
W. Chamberlain, A. John Blacker & Richard A. Bourne, Self-optimising reactive extractions:
towards the efficient development of multi-step continuous flow processes, Journal of Flow
Chemistry, Feb 2020.
Victor-Emmanuel H. Kassin, Thomas Toupy, Guillaume Petit, Pauline Bianchi, Elena Salvadeo & Jean-Christophe M.
Monbaliu, Metal-free hydroxylation
of tertiary ketones under intensified and scalable continuous flow conditions, Journal of Flow
Chemistry, Feb 2020.
Chetsada Khositanon, Kanyaporn Adpakpang, Sareeya Bureekaew & Nopphon Weeranoppanant, Continuous-flow purification of silver
nanoparticles and its integration with flow synthesis, Journal of Flow Chemistry, Feb
2020.
Govaerts, S., Nyuchev, A. & Noel, T., Pushing the
boundaries of C–H bond functionalization chemistry using flow technology, J Flow Chem,
Feb 2020.
Antimo Gioiello, Alessandro Piccinno, Anna Maria Lozza, and Bruno Cerra, The Medicinal Chemistry in the Era of Machines
and Automation: Recent Advances in Continuous Flow Technology, Journal of Medicinal
Chemistry, Feb 2020.
V Volker Hessel, Nam Nghiep Tran, Sanaz Orandi, Mahdieh Razi Asrami, Michael Evan Goodsite, Hung T. Nguyen,
Continuous-Flow Extraction of
Adjacent Metals – a Disruptive Economic Window for In-Situ Resource Utilization of Asteroids?,
Angewandte Chemie, Jan 2020.
Francesca Tentori, Elisabetta Brenna, Michele Crotti, Giuseppe Pedrocchi‐Fantoni, Maria Chiara Ghezzi and Davide
Tessaro, Continuous‐Flow
Biocatalytic Process for the Synthesis of the Best Stereoisomers of the Commercial Fragrances Leather
Cyclohexanol (4‐Isopropylcyclohexanol) and Woody Acetate (4‐(Tert‐Butyl)Cyclohexyl Acetate),
Catalysts, Jan 2020.
Edith Chow, Burkhard Raguse, Enrico Della Gasper, Steven J. Barrow, Jungmi Hong, Lee J. Hubble, Roger Chai, James S.
Cooper and Andrea Sosa Pintos, Flow-controlled
synthesis of gold nanoparticles in a biphasic system with inline liquid–liquid separation, React.
Chem.Eng., Jan 2020.
Nopphon Weeranoppanant and Andrea Adamo, In-Line Purification: A Key Component to
Facilitate Drug Synthesis and Process Development in Medicinal Chemistry, ACS Med. Chem.
Lett., Dec 2019.
Adam D. Clayton, Artur M.Schweidtmann, Graeme Clemens, Jamie A. Manson, Connor J.Taylor, Carlos G.Niñod Thomas
W.Chamberlain, Nikil Kapur, A. John Blacker, Alexei A.Lapkin, Richard A.Bourne, Automated self-optimisation of
multi-step reaction and separation processes using machine learning, Chemical Engineering
Journal, Nov 2019.
Le Sang, Jiacheng Tu, Han Cheng, Guangsheng Luo, Jisong Zhang, Hydrodynamics and mass transfer of
gas–liquid flow in micropacked bed reactors with metal foam packing, AIChE Journal,
Sep 2019.
Martina Letizia Contente Francesca Paradisi, Transaminase‐catalyzed continuous
synthesis of biogenic aldehydes, ChemBioChem, June 2019.
Martina Letizia Contente, Stefano Farris, Lucia Tamborini, Francesco Molinari, and Francesca Paradisi, Flow-based enzymatic synthesis of
melatonin and other high value tryptamine derivatives: a five-minute intensified process, Green
Chem., May 2019.
Luke Rogers and Klavs F. Jensen, Continuous manufacturing – the Green
Chemistry promise?, Green Chem., May 2019.
Zinia Jaman, Tiago J. P. Sobreira, Ahmed Mufti, Christina R. Ferreira, R. Graham Cooks, and David H. Thompson,
Rapid On-Demand Synthesis of Lomustine under
Continuous Flow Conditionss, Org. Process Res. Dev., Feb 2019.
René Lebl, David Cantillo, and C. Oliver Kappe, Continuous generation, in-line
quantification and utilization of nitrosyl chloride in photonitrosation reactions, React. Chem.
Eng., Jan 2019.
Matthew P. Thompson, Itziar Peñafiel, Sebastian C. Cosgrove, and Nicholas J. Turner, Biocatalysis Using Immobilized Enzymes in
Continuous Flow for the Synthesis of Fine Chemicals, Organic Process Research and
Development, Jan 2019.
Petra Martini, Andrea Adamo, Neilesh Syna, Alessandra Boschi, Licia Uccelli, Nopphon Weeranoppanant, Jack Markham,
and Giancarlo Pascali, Perspectives on the Use of Liquid
Extraction for Radioisotope Purification, Molecules, Jan 2019.
Jenny-Lee Panayides, Darren Lyall Riley, Rachel Chikwamba and Ian Strydom, Landscape and
opportunities for active pharmaceutical ingredient manufacturing in developing African economies,
Reaction Chemistry and Engineering, Jan 2019.
Samir Diab, Nikolaos Mytis, Andreas G.Boudouvis, and Dimitrios I.Gerogiorgis, Process Modelling, Design and
Technoeconomic Liquid-Liquid Extraction (LLE) Optimisation for Comparative Evaluation of Batch vs.
Continuous Pharmaceutical Manufacturing of Atropine,, Computers and Chemical
Engineering, Dec 2018.
Dr. Mathieu Lesieur, Dr. Claudio Battilocchio, Dr. Ricardo Labes, Dr. Jérôme Jac,q Dr. Christophe Genicot, Prof.
Steven V. Ley, and Dr. Patrick Pasau, Direct Oxidation of Csp3−H bonds using
in Situ Generated Trifluoromethylated Dioxirane in Flow, Chemistry, Nov 2018.
Clément Audubert, Alexanne Bouchard, Gary Mathieu, and Hélène Lebel, Chemoselective Synthesis of Amines from Ammonium
Hydroxide and Hydroxylamine in Continuous Flow, The Journal of Organic Chemistry, Oct
2018.
Kristina Søborg Pedersen, Joseph Imbrogno, Jesper Fonslet, Marcella Lusardi, Klavs F. Jensen and Fedor Zhuravlev,
Liquid–liquid
extraction in flow of the radioisotope titanium-45 for positron emission tomography applications,
Reaction Chemistry and Engineering, Oct 2018.
Anne-Catherine Bédard, Andrea Adamo, Kosi C. Aroh, M. Grace Russell, Aaron A. Bedermann, Jeremy Torosian, Brian Yue,
Klavs F. Jensen, and Timothy F. Jamison, Reconfigurable system for automated optimization
of diverse chemical reactions, Science, Sep 2018.
Carlos Mendoza Noémie Emmanuel Carlos Paez Laurent Dreesen Jean-Christophe M. Monbaliu, and Benoît Heinrichs,
Improving Continuous Flow Singlet
Oxygen Photooxygenations with Functionalized Mesoporous Silica Nanoparticles
ChemPhotoChem Aug 2018.
Eric Yu, Hari P R Mangunuru, Nakul S Telang, Caleb J Kong, Jenson Verghese, Stanley E Gilliland III, Saeed Ahmad,
Raymond N Dominey, and B Frank Gupton, High-yielding
continuous-flow synthesis of antimalarial drug hydroxychloroquine, Beilstein J Org
Chem. March 2018.
Hongwei Yang, Benjamin Martin, and Berthold Schenkel, On-Demand Generation and Consumption of
Diazomethane in Multistep Continuous Flow Systems, Organic Process Research &
Development March 2018.
Koichiro Masuda, Tomhohiro Ichitsuka, Nagatoshi Koumura, Kazuhito Sato, Shu Kobayashi, Flow fine synthesis with
heterogeneous catalysts, Tetrahedron Feb 2018.
Gemoets, H. P. L. (2018). Enabling and
accelerating C-H functionalization through continuous-flow chemistry Eindhoven: Technische Universiteit
Eindhoven
Jatuporn Salaklang, Veronique Maes, Matthias Conradi, Rudy Damsb and Tanja Junkers, Direct synthesis of
acrylate monomers in heterogeneous continuous flow processes Reaction Chemistry and
Engineering Dec 2017.
Joshua Britton and Timothy F Jamison, The assembly
and
use of continuous flow systems for chemical synthesis Nature Protocols Oct 2017.
Dr. Martina L. Contente, Federica Dall’Oglio, Dr. Lucia Tamborini, Prof. Francesco Molinari, Prof. Francesca
Paradisi, Highly Efficient Oxidation of Amines to Aldehydes with Flow-based
Biocatalysis
ChemCatChem Sep 2017.
Gabriel Glotz, Rene Lebl, Doris Dallinger, C. Oliver Kappe, Integration of Bromine and Cyanogen Bromide Generators for the Telescoped Continuous
Synthesis of Cyclic Guanidines Angew. Chem. Int. Ed. Sep 2017.
Amanda C. Wicker, Frank A. Leibfarth and Timothy F. Jamison, Flow-IEG enables programmable thermodynamic properties in sequence-defined unimolecular
macromolecules Polym. Chem. Sep 2017.
Valerio De Vitis, Federica Dall’Oglio, Andrea Pinto, Carlo De Micheli, Francesco Molinari, Paola Conti, Diego
Romano, Lucia Tamborini, Chemoenzymatic Synthesis in Flow
Reactors: A Rapid and Convenient Preparation of Captopril, Chemistry Open July 2017.
Michael R Chapman, Maria H. Kwan, Georgina King, Katherine E Jolley, Mariam Hussain, Shahed Hussain, Ibrahim E
Salama, Carlos González Nino, Lisa A Thompson, Mary E Bayana, Adam Clayton, Bao N. Nguyen, Nicholas J Turner, Nikil
Kapur, and
A. John Blacker, A Simple and Versatile
Laboratory Scale CSTR for Multiphasic Continuous-Flow Chemistry and Long Residence Times Org.
Process Res. Dev. June 2017.
Jinu Joseph John, SimonKuhn, Leen Braeken, and Tom Van Gerven, Temperature controlled interval
contact design for ultrasound assisted liquid-liquid extraction Chem. Eng. Res. Des.
June
2017.
Hannes P. L. Gemoets, Gabriele Laudadio, Kirsten Verstraete, Prof. Dr. Volker Hessel, and Dr. Timothy Noël,
A Modular Flow Design for the
meta-Selective C−H Arylation of Anilines Angew. Chem. Int. Ed. May 2017.
Nopphon Weeranoppanant, Andrea Adamo, Galym Saparbaiuly, Eleanor Rose, Christian Fleury, Berthold Schenkel, and
Klavs F. Jensen, Design of Multistage Counter-Current Liquid–Liquid Extraction for Small-Scale
Applications Ind. Eng. Chem. Res. Apr 2017.
Yi Shen, Nopphon Weeranoppanant, Lisi Xie, Yue Chen, Marcella R. Lusardi, Joseph Imbrogno, Moungi G. Bawendi and
Klavs F. Jensen, Multistage extraction platform for highly efficient and fully continuous
purification of nanoparticles Nanoscale Mar 2017.
C. Battilocchio, G. Iannucci, S. Wang, E. Godineau, A. Kolleth, A. De Mesmaeker and S. V. Ley Flow synthesis of cyclobutanones via [2 + 2] cycloaddition of keteneiminium salts and
ethylene gas React. Chem. Eng. Mar 2017.
Joshua Britton and Colin L. Raston, Multi-step continuous-flow synthesis Chem. Soc. Rev. Jan 2017.
David Cantillo, Bernd Wolf, Roland Goetz, and C. Oliver Kappe, Continuous Flow
Synthesis of a Key 1,4-Benzoxazinone Intermediate via a Nitration/Hydrogenation/Cyclization
Sequence Org. Process Res. Dev. Dec 2016.
Hansjoerg Lehmann, A scalable and safe continuous flow procedure for in-line generation of
diazomethane and its precursor MNU. Green Chemistry. Dec 2016.
Maryam Peer, Nopphon Weeranoppanant, Andrea Adamo, Yanjie Zhang, and Klavs F. Jensen, Biphasic catalytic hydrogen peroxide oxidation of alcohols in flow: Scale up and
extraction Org. Process Res. Dev. Aug 2016.
Franz J. Strauss, David Cantillo, Javier Guerrac and C. Oliver Kappe, A laboratory-scale continuous flow chlorine generator for organic synthesis
React. Chem. Eng. Aug 2016.
Baptiste Leforestiera and Markus Vögtle, Safe Generation and Direct Use of Chlorine Azide in Flow Chemistry: 1,2-Azidochlorination of
Olefins and Access to Triazoles Synlett. June 2016.
Andrea Adamo, Rachel L. Beingessner, Mohsen Behnam, Jie Chen, Timothy F. Jamison, Klavs F. Jensen, Jean-Christophe
M. Monbaliu, Allan S. Myerson, Eve M. Revalor, David R. Snead, Torsten Stelzer, Nopphon Weeranoppanant, Shin Yee
Wong, Ping Zhang, On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable
system
Science April 2016.
L Osorio-Planes, C Rodríguez-Escrich, MA Pericas, Removing the superfluous: a supported squaramide catalyst with a minimalistic linker
applied to the enantioselective flow synthesis of pyranonaphthoquinones Catalysis
Science & Technology Mar 2016.
Lorenzo Di Marco, Dr. Morgan Hans, Prof. Lionel Delaude and Dr. Jean-Christophe M. Monbaliu, Continuous-Flow N-Heterocyclic Carbene Generation and Organocatalysis
Chem. Eur. J. Feb 2016.
Javier Guerra, David Cantillo and C Oliver Kappe, Visible-Light Photoredox Catalysis using a Macromolecular Ruthenium Complex: Reactivity
and Recovery by Size-Exclusion Nanofiltration in Continuous Flow Catal. Sci.
Technol. Feb 2016.
Nicolas Lamborelle, Justine F Simon, Andre LUXEN and Jean-Christophe Monbaliu, Continuous-Flow Thermolysis for the Preparation of Vinylglycine Derivatives
Org. Biomol. Chem. Nov 2015.
Irina Sagamanova, Carles Rodríguez-Escrich, István Gábor Molnár, Sonia Sayalero, Ryan Gilmour, and Miquel A.
Pericàs, Translating the Enantioselective Michael Reaction to a Continuous Flow Paradigm with an
Immobilized, Fluorinated Organocatalyst ACS Catal. Sept 2015.
Leibfarth FA, Johnson JA, and Jamison TF, Scalable synthesis of sequence-defined, unimolecular macromolecules by
Flow-IEG
Proc. Natl. Acad. Sci. Aug 2015.
Chunhui Dai, David R. Snead, Ping Zhang, and Timothy F. Jamison, Continuous-Flow Syn and Purification of
Atropine with Sequential In-Line Separations of Structurally Similar Impurities J.
Flow Chem. July 2015.
Steffen Glöckner, Duc N. Tran, Richard J. Ingham, Sabine Fenner, Zoe E. Wilson, Claudio Battilocchio and Steven V.
Ley,
The rapid synthesis of oxazolines and their heterogeneous oxidation to oxazoles under
flow conditions Org. Biomol. Mol. Oct 2014.
Trevor A. Hamlin, Gillian M. L. Lazarus, Christopher B. Kelly, and Nicholas E. Leadbeater, A Continuous-Flow Approach to 3,3,3-Trifluoromethylpropenes:
Bringing Together Grignard Addition, Peterson Elimination, Inline Extraction, and Solvent
Switching. Org. Process Res. Dev. Aug 2014.
Andrea Adamo, Patrick L Heider, Nopphon Weeranoppanant, and Klavs F. Jensen, Membrane-Based, Liquid-Liquid Separator with Integrated
Pressure Control. Ind. Eng. Chem. Res. July 2013.
Jisong Zhang, Andrew R. Teixeira, Lars Thilo Kögl, Lu Yang, and Klavs F. Jensen Hydrodynamics of gas–liquid flow in
micropacked beds: Pressure drop, liquid holdup, and two-phase model AIChE
Journal June 2017.
Everett J. O’Neal, Chang Ho Lee, Julian Brathwaite, and Klavs F. Jensen, Continuous Nanofiltration and Recycle of an
Asymmetric Ketone Hydrogenation Catalyst ACS Catal. March 2015.
Reference to separator’s theory:
Kralj, J. G.; Sahoo, H. R.; Jensen, K. F., Integrated continuous microfluidic liquid-liquid extraction.
Lab Chip.2007, 7, (2), 256-263.
Reference to examples of use of liquid–liquid separators:
Sahoo, H. R.; Kralj, J. G.; Jensen, K. F., Multistep Continuous-Flow Microchemical Synthesis Involving Multiple
Reactions and Separations. Angew. Chem. Int. Ed. 2007, 46, (30), 5704-5708.
Cervera-Padrell, A. E.; Morthensen, S. T.; Lewandowski, D. J.; Skovby, T.; Kiil, S.; Gernaey, K. V., Continuous
Hydrolysis and Liquid–Liquid Phase Separation of an Active Pharmaceutical Ingredient Intermediate Using a Miniscale
Hydrophobic Membrane Separator. Org. Process Res. Dev. 2012, 16, (5), 888-900.
 |
||
 |
||
| Part Number | SEP-10 | |
|---|---|---|
| Width x Depth x Height | 77 mm (3.03 inch) x 29 mm (1.14 inch) x 71 mm (2.79 inch) | |
| Max Pressure | 2 MPa (290 psi) | |
| Ports | 1/4 – 28 flat bottom | |
| Wetted parts | Perfluorinated polymers (ETFA, PFA) | |
| Total Flow Rate | 0-10 ml/min | |
| Max Temperature | 90 ℃ | |
| Internal Volume | ~0.5ml | |
| Max Gas Flow Rate | ~100 sccm | |
*Scroll left to see more of the table
 |
||
 |
||
| Part Number | SEP—200 (SS/HS/FP) | |
|---|---|---|
| Width x Depth x Height | 206 mm (8.11 inch) x 26 mm (1.02 inch) x 196 mm (7.71 inch) | |
| Max Pressure | 2 MPa (290 psi) | |
| Ports | Swagelok for ¼‘’ OD | |
| Wetted parts | Model HS: Hastelloy C 276, PTFE, PFA, FFKM Model SS: SS 316, PTFE, PFA, FFKM Model FP: ETFE, PTFE, PFA, FFKM |
|
| Total Flow Rate | 20-200 ml/min | |
| Max Temperature | 130 ℃ for HS & SS, 90 ℃ for FP | |
| Internal Volume | ~35ml | |
| Max Gas Flow Rate | ~1000 sccm | |
*Scroll left to see more of the table
 |
||
 |
||
| Part Number | SEP—3000 (HS/SS) | |
|---|---|---|
| Width x Depth x Height | 460 mm (18.0 inch) x 150 mm (6.0 inch) x 607 mm (23.9 inch) | |
| Max Pressure | 1 MPa Standard – 2 MPa (290 psi) available upon request | |
| Ports | Swagelok 1/2’’ OD | |
| Wetted parts | Model HS: Hastelloy C 276, PTFE, PFA, FFKM Model SS: SS316, PTFE, PFA, FFKM |
|
| Total Flow Rate | 200-3000 ml/min | |
| Max Temperature | 130 ℃ | |
| Internal Volume | ~350ml | |
| Max Gas Flow Rate | ~10000 sccm | |
*Scroll left to see more of the table
Zaiput separators separate any process fluid containing more than 2 immiscible phases. This includes oil/water, aqueous/organic, organic/organic, gas/liquid and more. The Zaiput separators can be inserted into any continuous or batch process for inline or downstream separation.
Thanks to their special design with a metal shell, Zaiput separators can be operated at a high pressure, up to 290 psi/2 MPa. However, it is important to ensure that the pressures of the two outlets are not considerably different. Consult our technical support team for special use at high pressure.
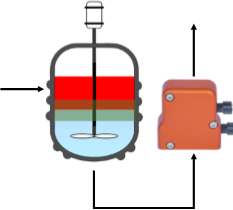
Separation in the Zaiput separator is an interfacial phenomena, not gravity-based. Therefore, the Zaiput separators can be used with any fluid mixture, regardless of their density difference.
Successful membrane separation requires an optimal pressure difference across the two sides of the membrane, i.e. retained and permeate sides. Zaiput separators incorporate an on-board pressure controller such that users no longer need to keep regulating the pressures of the two sides of the membrane. Click here to learn more about how it works.
Zaiput separators provide instantaneous, complete phase separation. They require neither a coalescing plate nor a settling tank. Their unique design eliminates the need for electronic apparatus for agitating parts or online process control. Additionally, they are very easy-to-use, inexpensive, and perfectly designed for a wide range of chemical environments (e.g., organic solvents, acidic solutions, high pressure).
All of the wetted parts inside the Zaiput separators are highly chemical-compatible. The metal shell does not contact the fluid. Zaiput separators can handle most organic solvents as well as corrosive solutions.
Temperature affects interfacial phenomena. Heating tends to decrease the interfacial tension of liquid mixtures. At very high temperatures, the liquid mixture is close to the miscibility region and may be difficult to separate. Contact us to consult our technical support team for special use with extreme temperatures.
Currently, we provide Zaiput separation devices in three versions, SEP-10, SEP-200 and SEP-3000. These versions cover flows ranging from 0 to 3000 mL/min.
The Zaiput separation devices separate immiscible fluids based on their wettability. This is different from filtration which uses a size-exclusion technique. Filtration generally removes solid from one liquid phase (either a pure liquid or a miscible liquid mixture).
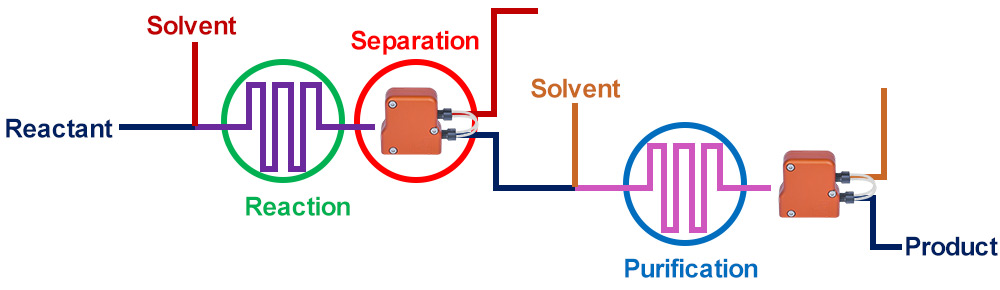
Zaiput’s separators can be used for any type of chemical process, in either the laboratory or in an industrial setting. They are suitable for high-value chemicals thanks to their very small dead volume and high efficiency. Below is a glimpse of potential applications:
The membranes used in the Zaiput separators are highly compatible with chemicals so it can function for weeks. However, depending on how clean the separated fluid is, the membrane may need to be replaced more frequently. This is especially true if the fluid contains a significant amount of solid particles/precipitates.