
Whether working in batch or flow, most chemists encounter challenges when performing difficult liquid-liquid/gas extractions. Zaiput Flow Technologies’ patented liquid-liquid/gas-liquid separators solve the most difficult separation problems with ease, backed by solid, proven results.
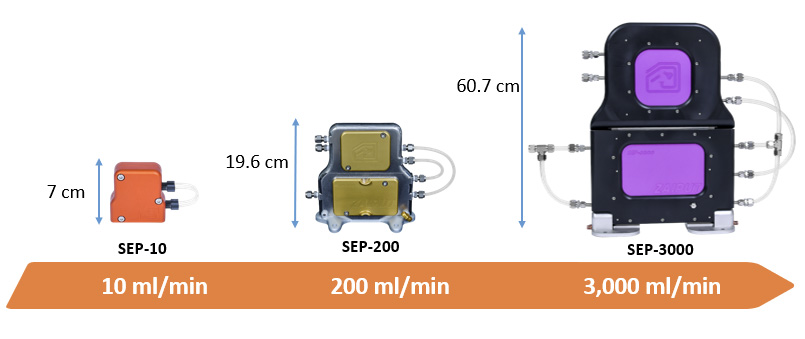
For larger scales separators click on this link: PRODUCTION SCALE SEPARATORS AND EXTRACTION PLATFORMS
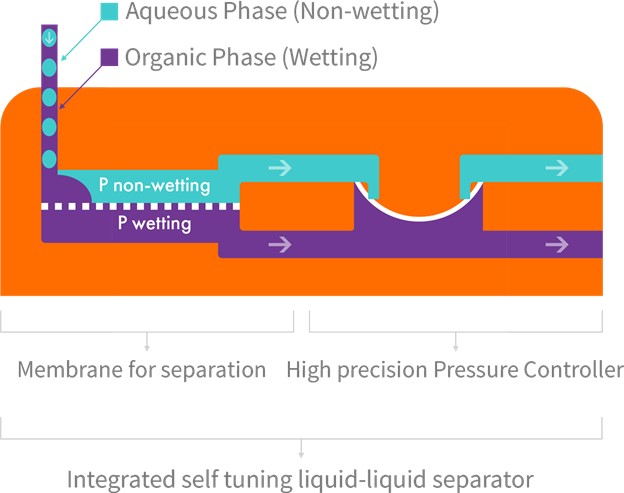
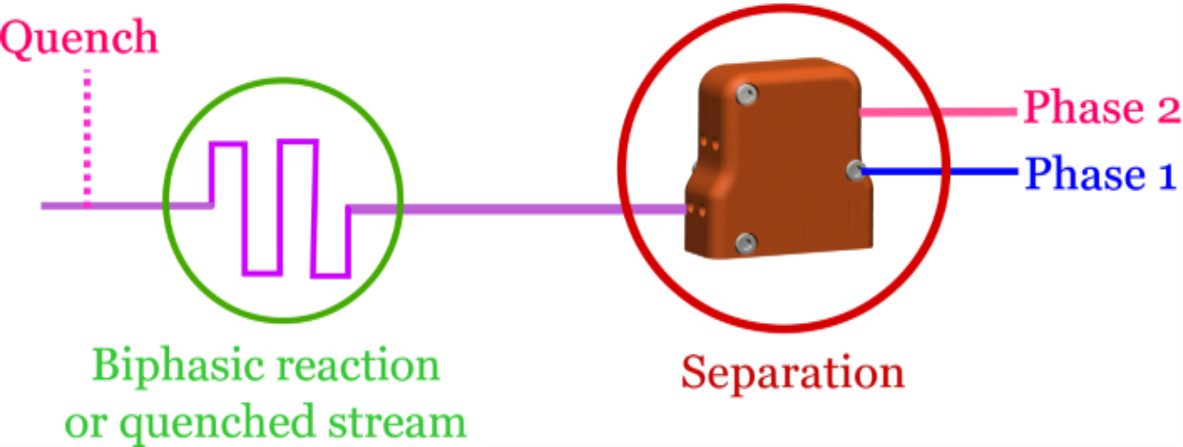
Zaiput Flow Technologies’ patented separators provide continuous separation of an immiscible phase (liquid-liquid or gas-liquid) by leveraging differences in wetting properties of the liquids onto a porous membrane:
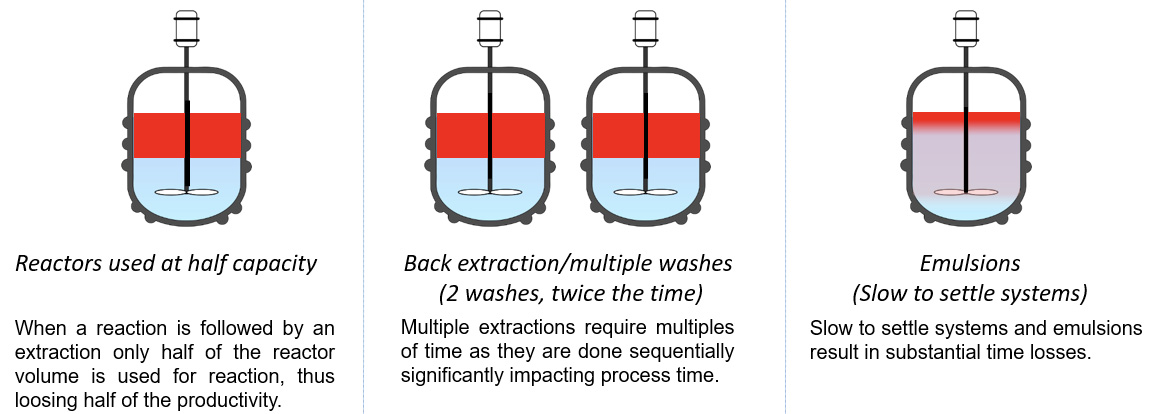
Our separators provide simple solutions to significant problems including: separation of emulsions, elimination of the need to run batches at half capacity and slow settling time, to name a few. Let’s look at the details of issues that may be encountered without our technology when working in batch or flow:
The modern approach to chemical manufacturing seeks to continuously leverage the advantages of continuous manufacturing
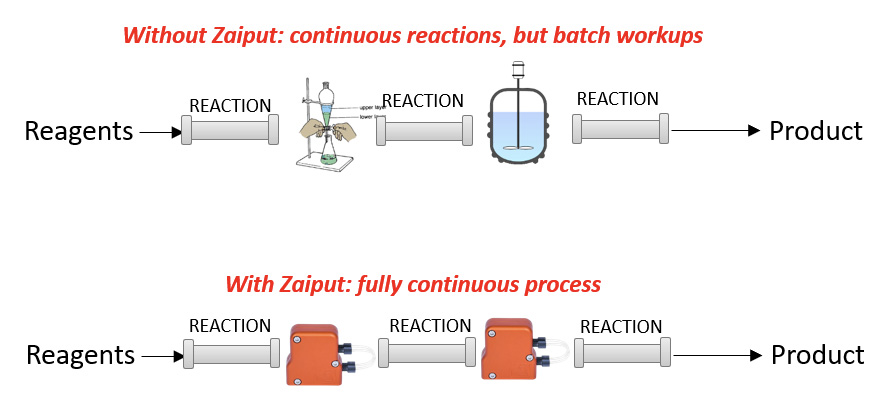
Before Zaiput separators existed, only a piecewise continuous process existed due to the lack of inline separation capabilities.
If the video does not play properly, click here to see our demo.
Zaiput Flow Technologies’ patented separators provide continuous separation of an immiscible phase (liquid-liquid or gas-liquid) by leveraging differences in wetting properties of the liquids onto a porous membrane.

Separator Function: When a hydrophobic membrane is in place, the organic, which is the wetting phase (purple), passes through the membrane (dotted line) while the aqueous, which is the non-wetting phase (blue), is retained.
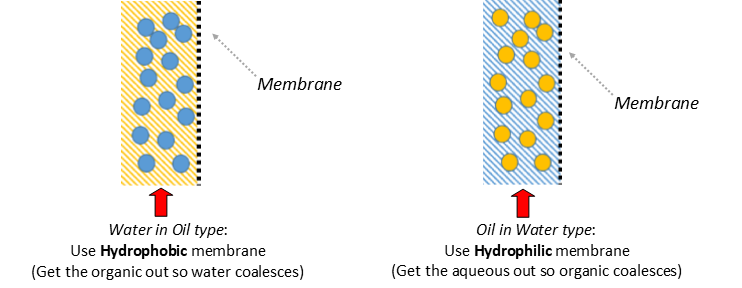
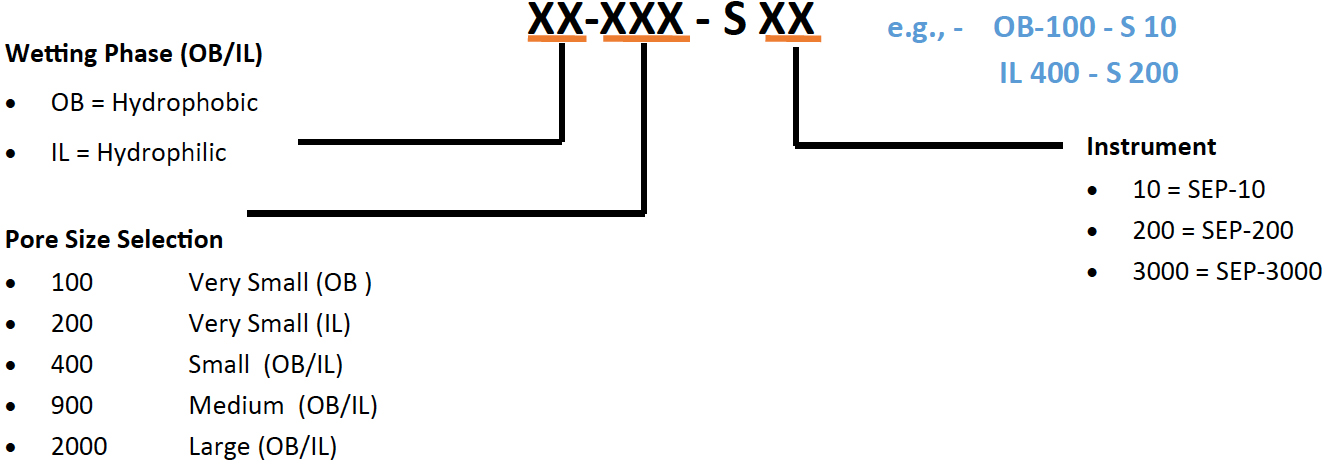
membrane separation can be done with either a hydrophilic or hydrophobic membrane
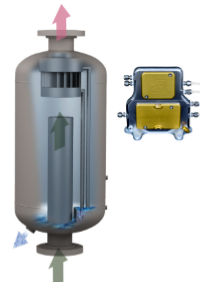
membrane-based liquid liquid separators require a much smaller footprint

A variety of membranes for your Zaiput separator are available to optimize phase separation performance and throughput. Membranes are available in both hydrophobic and hydrophilic, they are low cost and easy to replace.
Membrane selection process:
The key parameters for identifying a suitable membrane for phase separation are the interfacial tension between the two phases and the viscosity of the permeating phase (this has an effect on throughput of the device).
In general, the lower the interfacial tension, the smaller the pore size needs to be. However, smaller pore size reduces the maximum viscosity that can be accommodated by the membrane.
>> Membrane Sampler Package : MEM-10 (available on request for larger devices) – 7 packs of 10 membranes
>> Quick Start Package option : START-10 ( membrane samples MEM-10 + replacement diaphragm)
>> Specific membrane ordering info:
Contact us for the latest membrane offering
For general applications, we recommend reviewing the information in the Membrane Selection Guide.
If you have any questions, contact us at support@zaiput.com
 |
||
 |
||
| Part Number | SEP-10 | |
|---|---|---|
| Width x Depth x Height | 77 mm (3.03 inch) x 29 mm (1.14 inch) x 71 mm (2.79 inch) | |
| Max Pressure | 2 MPa (290 psi) | |
| Ports | 1/4 – 28 flat bottom | |
| Wetted parts | Perfluorinated polymers (ETFA, PFA) | |
| Total Flow Rate | 0-10 ml/min | |
| Max Temperature | 90 ℃ | |
| Internal Volume | ~0.5ml | |
| Max Gas Flow Rate | ~100 sccm | |
*Scroll left to see more of the table
 |
||
 |
||
| Part Number | SEP—200 (SS/HS/FP) | |
|---|---|---|
| Width x Depth x Height | 206 mm (8.11 inch) x 26 mm (1.02 inch) x 196 mm (7.71 inch) | |
| Max Pressure | 2 MPa (290 psi) | |
| Ports | Swagelok for ¼‘’ OD | |
| Wetted parts | Model HS: Hastelloy C 276, PTFE, PFA, FFKM Model SS: SS 316, PTFE, PFA, FFKM Model FP: ETFE, PTFE, PFA, FFKM |
|
| Total Flow Rate | 20-200 ml/min | |
| Max Temperature | 130 ℃ for HS & SS, 90 ℃ for FP | |
| Internal Volume | ~35ml | |
| Max Gas Flow Rate | ~1000 sccm | |
*Scroll left to see more of the table
 |
||
 |
||
| Part Number | SEP—3000 (HS/SS) | |
|---|---|---|
| Width x Depth x Height | 460 mm (18.0 inch) x 150 mm (6.0 inch) x 607 mm (23.9 inch) | |
| Max Pressure | 1 MPa Standard – 2 MPa (290 psi) available upon request | |
| Ports | Swagelok 1/2’’ OD | |
| Wetted parts | Model HS: Hastelloy C 276, PTFE, PFA, FFKM Model SS: SS316, PTFE, PFA, FFKM |
|
| Total Flow Rate | 200-3000 ml/min | |
| Max Temperature | 130 ℃ | |
| Internal Volume | ~350ml | |
| Max Gas Flow Rate | ~10000 sccm | |
*Scroll left to see more of the table
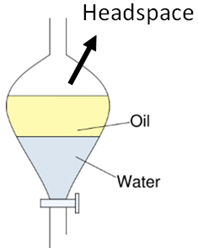
Zaiput separators separate any process fluid containing more than 2 immiscible phases. This includes oil/water, aqueous/organic, organic/organic, gas/liquid and more. The Zaiput separators can be inserted into any continuous or batch process for inline or downstream separation.
Thanks to their special design with a metal shell, Zaiput separators can be operated at a high pressure, up to 290 psi/2 MPa. However, it is important to ensure that the pressures of the two outlets are not considerably different. Consult our technical support team for special use at high pressure.
Separation in the Zaiput separator is an interfacial phenomena, not gravity-based. Therefore, the Zaiput separators can be used with any fluid mixture, regardless of their density difference.
Successful membrane separation requires an optimal pressure difference across the two sides of the membrane, i.e. retained and permeate sides. Zaiput separators incorporate an on-board pressure controller such that users no longer need to keep regulating the pressures of the two sides of the membrane. Click here to learn more about how it works.
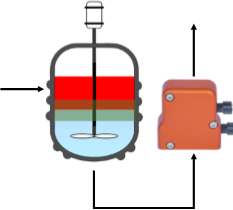
Zaiput separators provide instantaneous, complete phase separation. They require neither a coalescing plate nor a settling tank. Their unique design eliminates the need for electronic apparatus for agitating parts or online process control. Additionally, they are very easy-to-use, inexpensive, and perfectly designed for a wide range of chemical environments (e.g., organic solvents, acidic solutions, high pressure).
All of the wetted parts inside the Zaiput separators are highly chemical-compatible. The metal shell does not contact the fluid. Zaiput separators can handle most organic solvents as well as corrosive solutions.
Temperature affects interfacial phenomena. Heating tends to decrease the interfacial tension of liquid mixtures. At very high temperatures, the liquid mixture is close to the miscibility region and may be difficult to separate. Contact us to consult our technical support team for special use with extreme temperatures.
Currently, we provide Zaiput separation devices in three versions, SEP-10, SEP-200 and SEP-3000. These versions cover flows ranging from 0 to 3000 mL/min.
The Zaiput separation devices separate immiscible fluids based on their wettability. This is different from filtration which uses a size-exclusion technique. Filtration generally removes solid from one liquid phase (either a pure liquid or a miscible liquid mixture).
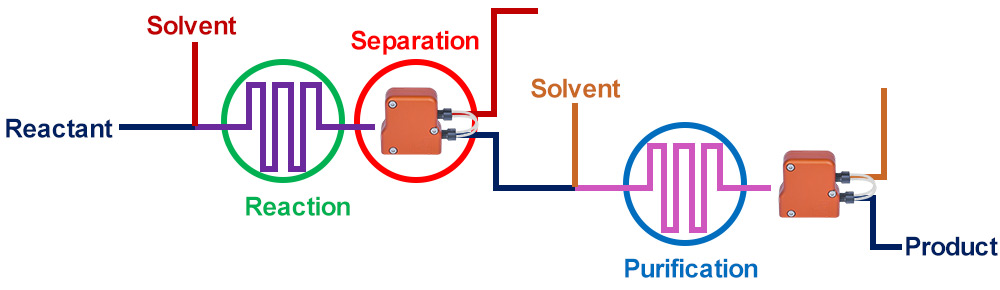
Zaiput’s separators can be used for any type of chemical process, in either the laboratory or in an industrial setting. They are suitable for high-value chemicals thanks to their very small dead volume and high efficiency. Below is a glimpse of potential applications:
The membranes used in the Zaiput separators are highly compatible with chemicals so it can function for weeks. However, depending on how clean the separated fluid is, the membrane may need to be replaced more frequently. This is especially true if the fluid contains a significant amount of solid particles/precipitates.